Stay Informed on the New Regulation! Production – Import – Sale of Category 5 Narcotics (excluding cannabis and hemp)

Stay Informed on the New Regulation!
Production Import Sale of Category 5 Narcotics (excluding cannabis and hemp)
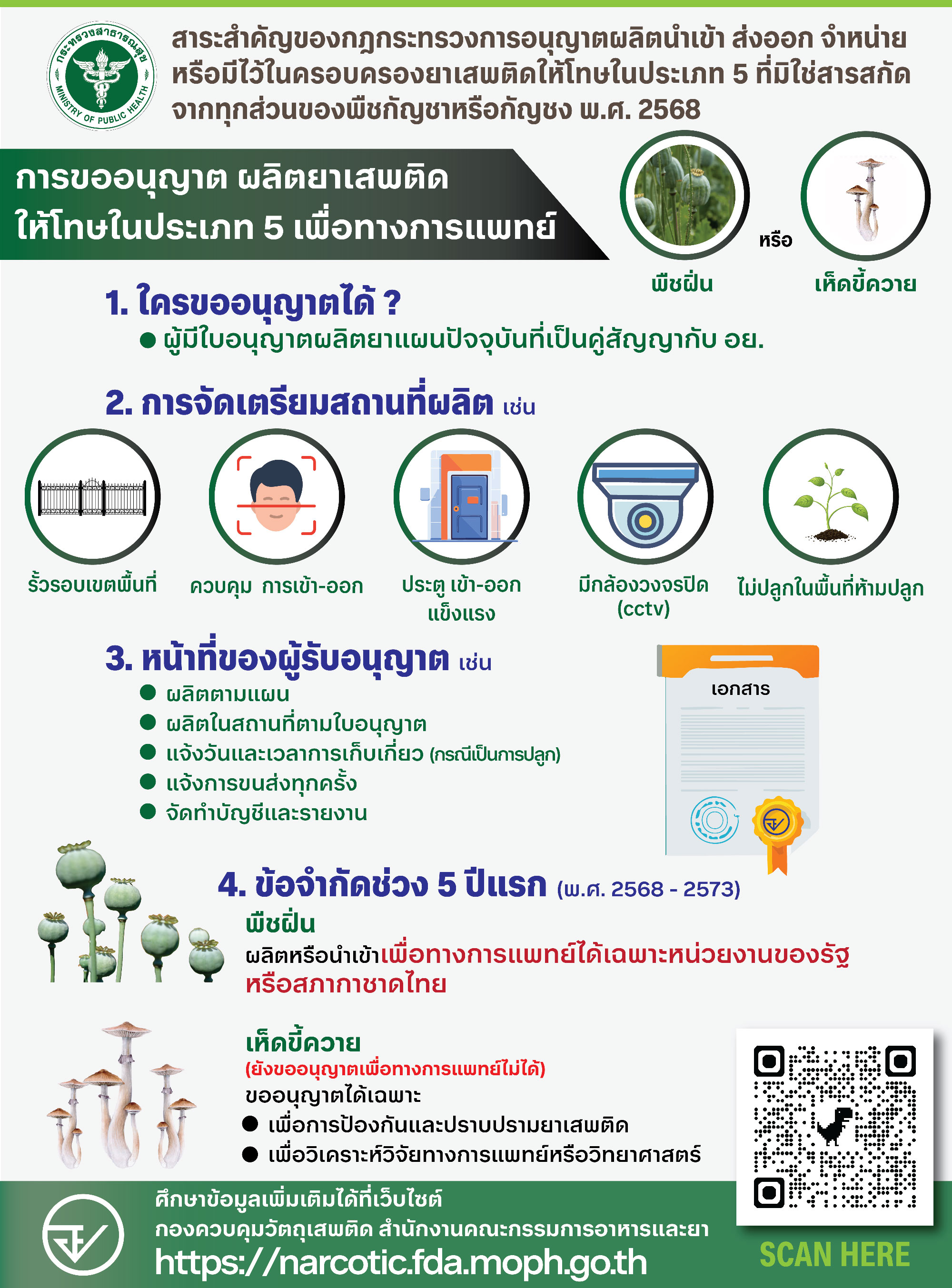
Key points of the Ministerial Regulation on the licensing of production, import, export, sale, or possession of Category 5 narcotics (excluding extracts from all parts of cannabis or hemp), B.E. 2568 (2025)
Applying for a License to Produce Category 5 Narcotics for Medical Purposes
(Opium poppy or Psilocybin mushrooms)
1. Who can apply?
Holders of a pharmaceutical production license who are under contract with the Food and Drug Administration (FDA).
2. Requirements for production facilities include:
Secure perimeter fencing
Controlled entry and exit
Strong entrance and exit gates
CCTV surveillance
Not located in prohibited cultivation zones
3. Duties of license holders:
Produce according to the approved plan
Produce only at the licensed site
Notify the authority of harvest date and time (in case of cultivation)
Notify every instance of transportation
Maintain proper records and submit reports
4. Restrictions during the first 5 years (2025 - 2030):
Opium poppy:
Production or import for medical purposes permitted only for government agencies or the Thai Red Cross Society.
Psilocybin mushrooms:
(Medical use licenses are not yet permitted.)
Licenses can be granted only for:
Drug prevention and suppression purposes
Medical or scientific research and analysis
For more details, visit the Narcotics Control Division, Food and Drug Administration:
https://narcotic.fda.moph.go.th
Source: Narcotics Control Division, Food and Drug Administration
https://www.fda.moph.go.th/news-other/news-02090668



